The results of the UK Prospective Diabetes Study (UKPDS) changed the face of diabetes treatment, addressing fundamental questions about the condition, the answers to which still underpin the management of people with diabetes today. And it was a mammoth undertaking, spawning 93 original research papers to date, far exceeding the trialists’ initial expectations for duration and cost, and requiring them to invent now standard concepts for the running of large clinical trials.
Twenty years after the publication of the primary findings, medwireNews talks to longtime UKPDS investigator Professor David Matthews (University of Oxford, UK) about the struggles of the team to bring the trial to completion and the impact of the findings on diabetes care.
A limited armamentarium
It is hard to overstate just how different the diabetes treatment landscape was back in 1976 when Robert Turner, of the University of Oxford, first scribbled his plan for the UKPDS on a piece of paper, while confined to a hotel room in Delhi by diarrhea.
“In the way that the DCCT had the same impact round the world on type 1 diabetes, the UKPDS had instantaneous impact round the world,” says Matthews, describing the trial as “absolutely fundamental.”
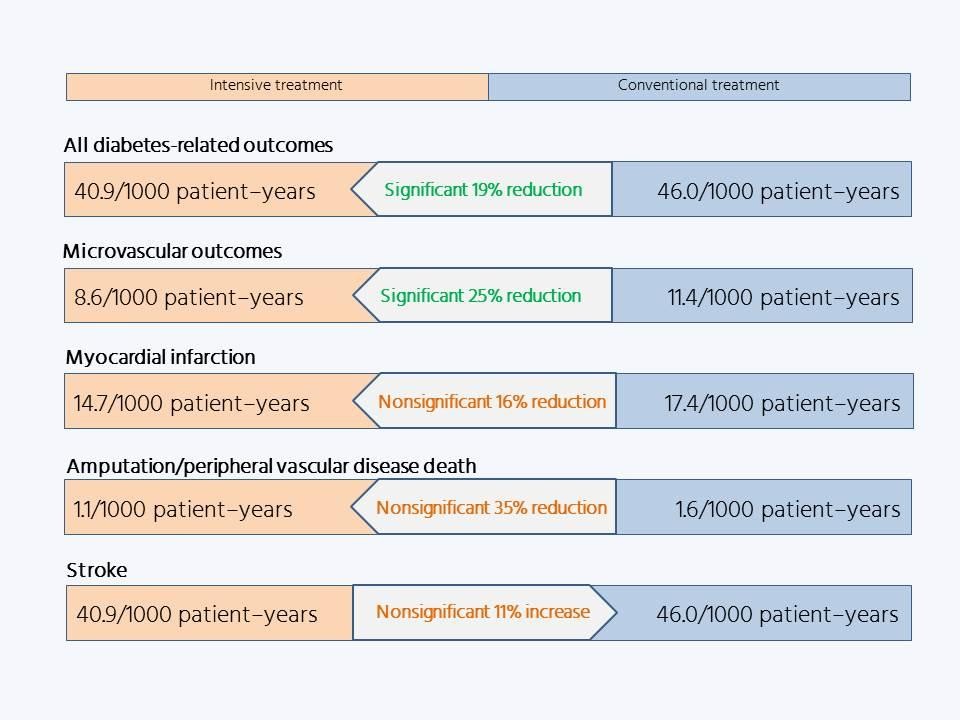
The UKPDS, which published its primary findings in 1998, provided the first evidence that controlling people’s blood sugar reduced their risk for developing micro- and macrovascular complications.
“Until the UKPDS reported there was some doubt about that,” says Matthews. “Indeed, the people who trained me in Oxford said, well, I like to let the diabetes rip for a bit because you lose weight, because you’re losing all this glucose in your urine and so on.
“And, of course, none of that now would be regarded as ethical, and all of that is down to the UKPDS.”
Over the few years preceding the trial’s launch, the UGDP study had published, amid controversy [1]. Its results (some of which were leaked in the press before the official presentation) suggested that treatment with the sulfonylurea tolbutamide or the biguanide phenformin might increase the risk for, respectively, cardiovascular mortality and all-cause mortality, and that insulin treatment was of no benefit. But at the time, these were the only medications available in the UK for treating people with type 2 diabetes.
“There was nothing else really around,” says Matthews. “We’ve now got about 20 different agents you could use.”
So the UKPDS randomly assigned patients to receive intensive treatment with a sulfonylurea (chlorpropamide, glibenclamide, or glipizide) or insulin, or conventional treatment in the form of dietary intervention, and those who were more than 120% of their ideal bodyweight were additionally randomized to receive or not receive metformin.
UKPDS Group. Lancet 1998; 352: 837–853
The aim of the pharmacologic treatment was to achieve intensive control of patients’ blood glucose (fasting blood glucose <6 mmol/L), and in 1987 an embedded study was launched to assess the effects of keeping patients’ blood pressure under control, using captopril or atenolol. But the targets used look very unfamiliar to people accustomed to current medical care; for example, the intensive and relaxed blood pressure targets were 150/85 and 180/105 mmHg, respectively.
“You couldn’t possibly run a trial now with 180 [mmHg] as some sort of threshold, or indeed 15 mmol/L of glucose, which is the UKPDS threshold for rescue,” observes Matthews.
A long haul
The investigators found that blood glucose deteriorated over time despite the various treatments, and so amended the protocol to allow intensification of medication if blood glucose exceeded 6 mmol/L in the intensive treatment group and 15 mmol/L in the conventional group.
There were many such amendments required during the course of the UKPDS, caused in part by the investigators, according to Matthews, “not understanding how long you would need to run the wretched thing for.”
He says that “one of the great triumphs of the UKPDS was to keep it funded for 20 years from the time of inception to the time that it had finished,” calling it “a remarkable story and a story of perseverance.”
“And Robert [Turner] sent at least his own weight in paper to various authorities trying to fund it,” he adds.
The first patient was recruited to the pilot trial in 1977, but the recruitment period lasted for 10 years and the primary findings were not published until 1998. Matthews says that, ideally, such a trial would have a 6-month recruitment period to obtain a “coherent” cohort of patients, enrolled against the same clinical practice background.
“The median dwell time within the UKPDS had been 10 years, but some people had been in for 20,” he says. “The problem with trials like that is that the investigators die before the patients.”
Matthews explains that modern trials, benefitting from the experience gained from trials such as the UKPDS, enroll patients at high risk for the endpoint events of interest, whereas the UKPDS is the only large trial in diabetes to enroll newly diagnosed patients who were otherwise healthy.
“And by entirely healthy, I mean that we excluded people for instance who’d got cancer as well, or any overt heart disease, with the result in fact that in the first 2 years of the UKPDS nobody died, scarcely nothing happened, to the great frustration, of course, of the people doing trials who were desperate for things to happen.
“I mean that’s what you want to happen in trials; you don’t know what treatment [the patients are] on, but you do want events.”
Thus, the planned 5–6-year follow-up became a 10-year follow-up, during the course of which various sulfonylureas waxed and waned in popularity, acarbose came onto the market (and was duly included in a UKPDS substudy), and statins were introduced.
“So the environmental background against which the UKPDS was finally announced had changed a lot from where it was at inception,” says Matthews, although he notes that the “explosion” in novel diabetes medications occurred after the UKPDS reported.
One potential problem with accumulating knowledge over a long clinical trial is investigators beginning to suspect that blood glucose control would turn out to be beneficial, and adjusting the management of their patients accordingly. Although “damnable” in clinical trials, Matthews concedes it is unavoidable in one lasting 20 years.
“People start to game the trial on the basis of saying, actually, I don’t want my patients running at 15 mmol/L. Whatever arm they’re in I’m not going to allow that to happen, so they just tighten everything up.”
He adds: “I know of at least one person where I became slightly sensitized to what was going on in their clinic – someone I have a very high regard for incidentally.”
Nevertheless, the actions of a few individuals, relatively late in the course of a very large trial, would be unlikely to influence its overall result. Indeed, any efforts of investigators to achieve better glucose control in the conventional treatment group would serve only to diminish the differences with the intensive treatment group, and reduce the likelihood of a positive result, which the UKPDS achieved regardless.
Keeping the show on the road
As well as dealing with changes in medicine over the course of the UKPDS, the investigators also had to cope with changes in technology.
“There’s a whole load of stuff that starts off on huge charts that are stuck on the wall and colored in, the squares being colored in when people come through to the clinics,” says Matthews. Over time this advanced to card indexes and then to computers.
“Then there’s the whole thing about what are you measuring,” he says.
The team started off measuring patients’ fasting blood glucose, because the trial’s launch predated the routine use of glycated hemoglobin. “You see lots of UKPDS data that’s got hemoglobin A1c as the axis, one of the axes, but actually that’s converted from fasting plasma glucose for a whole lot of the early stuff.”
Complicating that is the fact that assays are continually changed and improved over time “and so you start off measuring something one way and then you measure it another way and then you do a back correction and so on,” says Matthews. “So there were lots and lots and lots of meetings about all of this stuff – about how you maintain the integrity of the data and so on.”
Managing such a large and lengthy trial also required the on-the-spot invention of concepts now considered commonplace.
“I did all of the endpoints, for instance, in the UKPDS,” says Matthews. “And so we started off, you know, writing down what the endpoints were, but then realized you need a committee and then you need a data-monitoring committee, an IDMC [independent data monitoring committee], and all of these things that were invented on the hoof.”
He says: “The remarkable thing is that Robert and Rury [Holman] and all of that team managed over the years to keep the whole thing chugging on and that the UKPDS is as robust as it is.”
Robert Turner, sadly, played no part in the longer-term follow-up of the UKPDS, dying unexpectedly of a stroke in 1999.
“The tragedy that Robert didn’t live more than a year beyond the UKPDS being published is a huge tragedy because [his] knowledge about how and why you run trials and so on was something that was a learning curve over 20 years, and to some extent that is lost,” says Matthews.
“And I mean Robert was a lovely person, and with a great sense of humor about things, and never gave up.”
Lessons and legacy
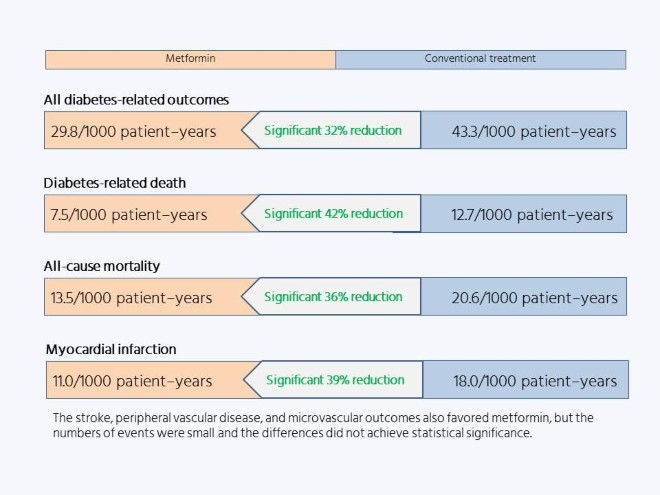
These days, metformin is the first-line diabetes medication of choice worldwide, regardless of bodyweight. Yet Matthews says that there were no further randomized trials of metformin after the UKPDS, so the evidence for its efficacy rests entirely on the results of the 342 overweight people assigned to take it in the UKPDS, and the 951 and 411 overweight controls assigned to intensive or conventional treatment without metformin, respectively.
UKPDS Group. Lancet 1998; 352: 854–865 2021 Springer Healthcare Limited. Part of the Springer Nature Group.
But the addition of metformin in the UKPDS resulted in marked risk reductions, of a third or more, compared with dietary treatment, which Matthews describes as “a very clear outcome,” despite the relatively small size of the substudy.
“It’s like having a gun, where if I fire it six times and six people die that’s probably enough to demonstrate the gun works,” he says. Small numbers become an issue only when the effect of the intervention is smaller, and therefore requires greater statistical power to detect it.
In addition, metformin does not give its users hypoglycemia, and “it’s dirt cheap, absolutely dirt cheap,” says Matthews.
“It lowers your blood sugar, and it stops you getting complications. I mean what more do you want from a drug?
“The answer is, obviously, I’d like to know how it works!” he says, pointing out that its mechanisms remain elusive, despite the length of time the medication has been in use.
With the exception of metformin, the oral medications used in the UKPDS are now barely used, and the market is awash with novel diabetes treatments. None of which diminishes the importance of the trial because, as Matthews stresses, “the setup of the UKPDS was about glucose control and not how you might do it.”
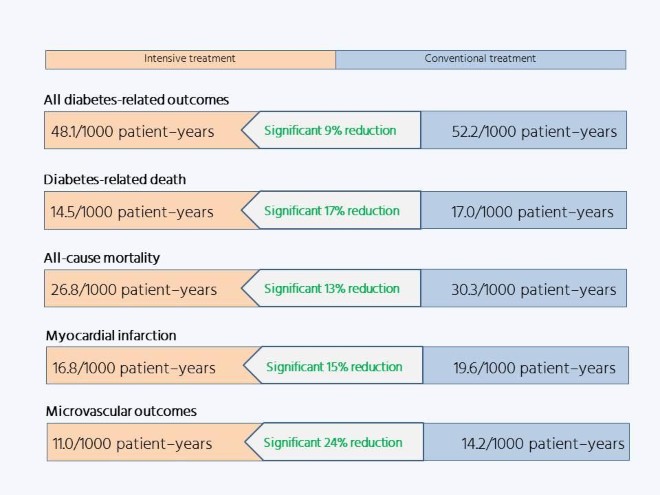
The importance of this strategy is underlined by the results of the long-term follow-up. The impact of the UKPDS findings ensured that blood glucose control became standard practice for patients with type 2 diabetes, so the blood glucose differences between the two groups were rapidly lost. Yet 10 years after the conclusion of the randomized phase of the trial, the investigators found a persistent significant difference in microvascular outcomes favoring patients who had received intensive treatment and, moreover, the formerly not-quite-significant difference in macrovascular outcomes had achieved statistical significance: the legacy effect.
Holman R, et al. N Engl J Med 2008; 359: 1577–1589 2021 Springer Healthcare Limited. Part of the Springer Nature Group.
The DCCT, was initiated after the UKPDS but reported earlier, and found a similar effect among patients with type 1 diabetes, which the investigators dubbed “metabolic memory.” But Matthews says that it is “a misnomer to call it metabolic memory because your metabolism does not remember anything.”
The concept, he says, is “flawed” because patients in the intensive care group in the DCCT “had people talking to them several times a week and they had dietetics and they had people on the telephone and all the rest for 4 years and more.”
The long-term effects of the DCCT were therefore simply about memory per se, he believes, with the intensive treatment group, having been rigorously trained in self-management, retaining this knowledge after the conclusion of the randomized treatment period.
“Metabolic memory is a slightly crazy term,” says Matthews. He feels legacy to be a better description, encompassing the notion of something that has finished but leaves something for the future. “So we dreamt up the word legacy that we’re pleased with and [is] so much better – a much better concept and allows one to differentiate these things.”
The existence of the legacy effect has more implications for clinical practice than simply the need to control blood glucose in diagnosed patients, by demonstrating the importance of early diagnosis and treatment, before complications start to take hold.
“And interestingly, of course, early on in type 2 diabetes you’ve got a better chance of getting it under control because your insulin-producing cells, the beta cells, are working probably about 50% of normal function, whereas 10 years later they’re running at about 15%, so it’s much more difficult to control,” says Matthews.
“So the early years are an absolute gift to physicians to say, look you’ve got to really – you can delay the onset of complications by a significant amount of time if you get on and treat these people early.”
The legacy effect applied only to blood glucose control, however; the period of intensive blood pressure control had no influence on patients’ outcomes 10 years after its conclusion.
Matthews says: “I’ve always used the analogy of an old, rusty car that just about goes to say, look, the rust on the car is the result of things that happened a long time ago and that’s like the glucose – you know, the way you’ve looked after the bodywork is going to have an effect now – but that the condition of the air in the tire is related to what’s happened recently and that’s like blood pressure.
“And you can sort of come out of that by saying, look, if you forgot your [antidiabetes medications] for a couple of weeks while you were on holiday if wouldn’t matter, but if you forgot your blood pressure tablets then you’d better get along to the pharmacy and make sure that you’ve got those because otherwise you’re at considerable risk.”
A wealth of data
These headline findings barely scratch the surface of the new knowledge obtained from studying the UKPDS cohort. The trial has so far generated a whopping 93 original research papers, covering subjects as diverse as healthcare costs and genetics, although Matthews notes that they are “all much smaller print than the big messages.” However, he highlights the epidemiologic paper, published in The BMJ in 2000, that demonstrates the close association between glycated hemoglobin and outcomes, irrespective of treatment group [2].
The wealth of data over the years is “extraordinary,” he says, “and I can’t speak highly enough of Rury keeping all this stuff going and having the statisticians to keep these data alive and true.”
By Eleanor McDermid