Author: Lynda Williams
medwireNews: The likelihood of successful globe salvage for children with advanced unilateral retinoblastoma is higher with intra-arterial chemotherapy than with a conventional intravenous approach, randomised trial findings suggest.
“Intra-arterial chemotherapy could be an acceptable first-line treatment” for this patient population, say Xianqun Fan, from Shanghai Jiao Tong University School of Medicine in China, and co-workers in The Lancet Child & Adolescent Health.
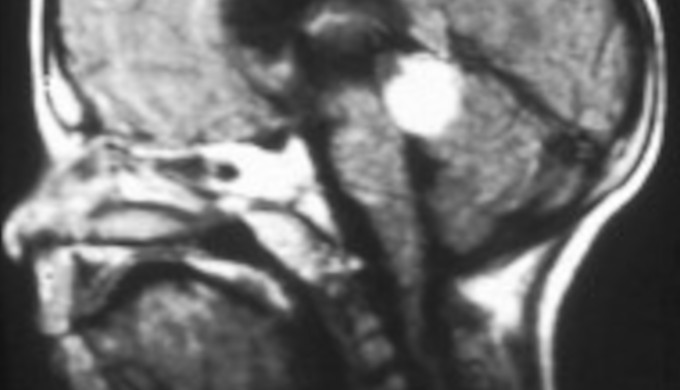
The open-label study, performed at six Chinese hospitals, included 234 children, aged a median 23.6 months, with new-onset unilateral group D or E retinoblastomas on the Philadelphia version of the International Intraocular Retinoblastoma Classification. All of the retinoblastomas were considered poorly defined, large or very large without high-risk features.
For the study, 72 patients (44% girls) were randomly assigned to receive at 4-week intervals four cycles of intra-arterial melphalan 0.5–7.5 mg/kg as determined by age, plus carboplatin 20 mg on the first and third cycle and topotecan 1 mg on the second and fourth cycles. Treatment was given under general anaesthetic and patients were also given two doses of subcutaneous nadroparin 0.1 mL at 12-hour intervals after each round.
A further 71 patients (61% girls) instead received six cycles of intravenous vincristine 0.05 mg/kg (<36 months old) or 1.5 mg/m2 (≥36 months old), etoposide 5.0 mg/kg or 150 mg/m2 and carboplatin 18.6 mg/kg or 560 mg/m2. Both treatment arms also received cryotherapy or laser therapy for small tumours as required.
After a median 35.8 months of follow-up, the median progression-free globe salvage time was 18.4 months with intra-arterial therapy and 8.9 months with intravenous therapy. The 2-year progression-free globe salvage rates were a corresponding 53% and 27%, giving a significant rate ratio of 1.97 in favour of the intra-arterial regimen.
There was no significant difference in the overall survival of the two treatment arms, with 96% of both treatment arms alive at time of analysis.
All six patients who died had group E retinoblastoma, with three and two deaths in the intra-arterial and intravenous groups, respectively, from intracranial metastasis via the optic nerve following refusal of enucleation. The sixth death from distant metastasis after enucleation occurred in a patient given intravenous therapy.
Objective responses were a significant 1.17 times more common with intra-arterial than intravenous chemotherapy, at rates of 97% versus 83%, respectively.
Post-hoc analysis showed a comparable rate of local relapse in the intra-arterial and intravenous arms (17 vs 18%) and a similar rate of globe salvage after local relapse was achieved in these patients (67 vs 62%).
The most common adverse (AE) in both treatment arms was myelosuppression, occurring significantly less frequently with intra-arterial than intravenous chemotherapy (51 vs 70%); most of these events were at grade 1 or 2, with grade 3 rates of 7% and 13%, respectively, and 1% at grade 4 in both groups.
Local AEs in the intra-arterial and intravenous groups included chorioretinal atrophy (33 vs 19%), cataract (21 vs 27%), vitreous haemorrhage (19 vs 15%), ptosis (17 vs 11%), and redness (13 vs 11%),
In addition, 18% of patients given intra-arterial chemotherapy experienced ophthalmic artery stenosis and 3% had occlusion, the latter of which Fan et al acknowledge “can cause irreversible vision loss”, but the investigators note that most patients had “already lost vision, so the main objective of treatment was to save lives and conserve the eye.”
The author of a linked comment says that the “chief concerns” about the use of intra-arterial chemotherapy are the lack of “systemic protective effect against micrometastastic disease” and the “technical challenges” of the procedure in low-volume hospitals.
Guillermo Chantada (Hospital Sant Joan de Déu, Barcelona, Spain) therefore cautions that the current findings do not yet “support the conclusion that intra-arterial chemotherapy should become the standard of care for advanced unilateral intraocular retinoblastoma worldwide.”
But he believes that for low- and middle-income countries where enucleation is the standard of care and surgery refusal can lead to treatment abandonment, “intra-arterial chemotherapy could probably save eyes and lives”, albeit at the cost of more intensive follow-up than enucleation.
Chantada concludes: “Improving access to intra-arterial chemotherapy should be considered an important step towards achieving the 2030 target” of increasing the global survival rate to at least 60%.
News stories are provided by medwireNews, which is an independent medical news service provided by Springer Healthcare Ltd. © 2023 Springer Healthcare Ltd, part of the Springer Nature Group
This independent news story was supported by an educational grant from L’Institut Servier, Suresnes, France.
Lancet Child Adolesc Health 2023; doi:10.1016/S23524642(23)00414-4
Lancet Child Adolesc Health 2023; doi: 10.1016/S2352-4642(23)00165-7
Image Credits: © Aerts, I, Lumbroso-Le Rouic, L, Marion Gauthier-Villars, M, Brisse, H, Doz, F, Desjardins, L. via Wikimedia Commons